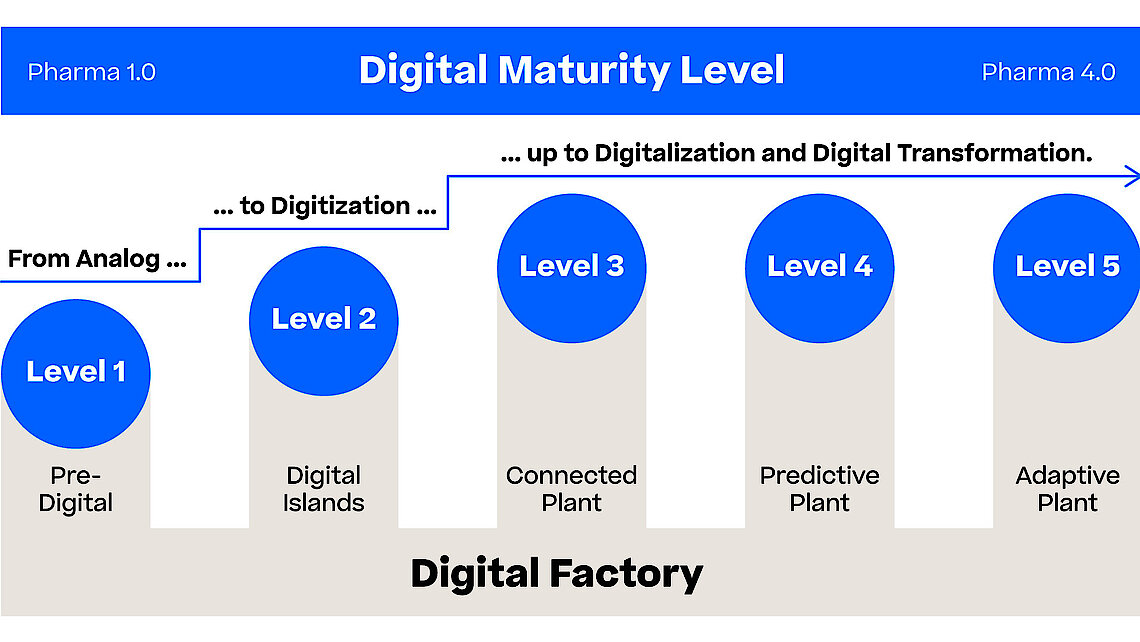
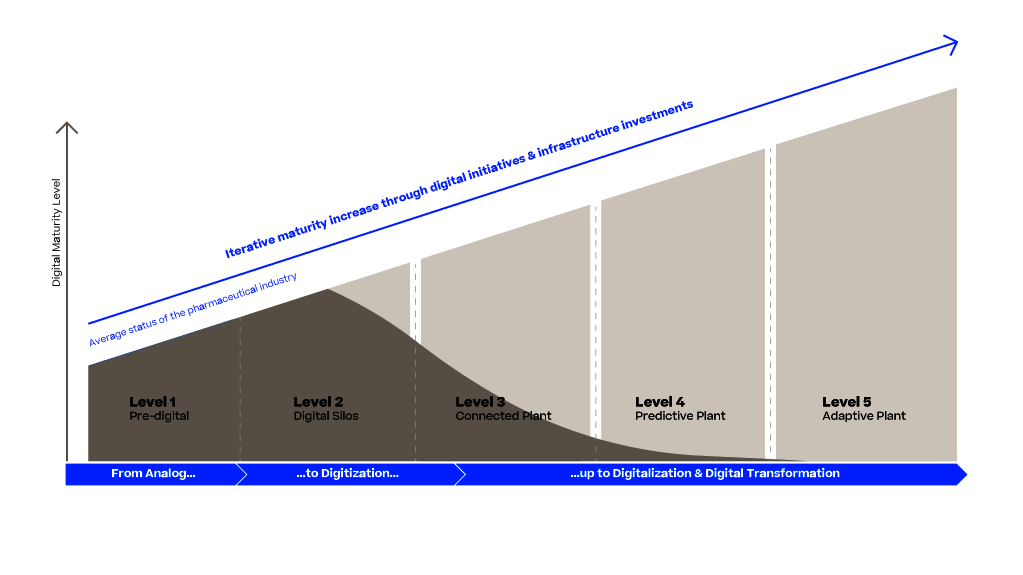
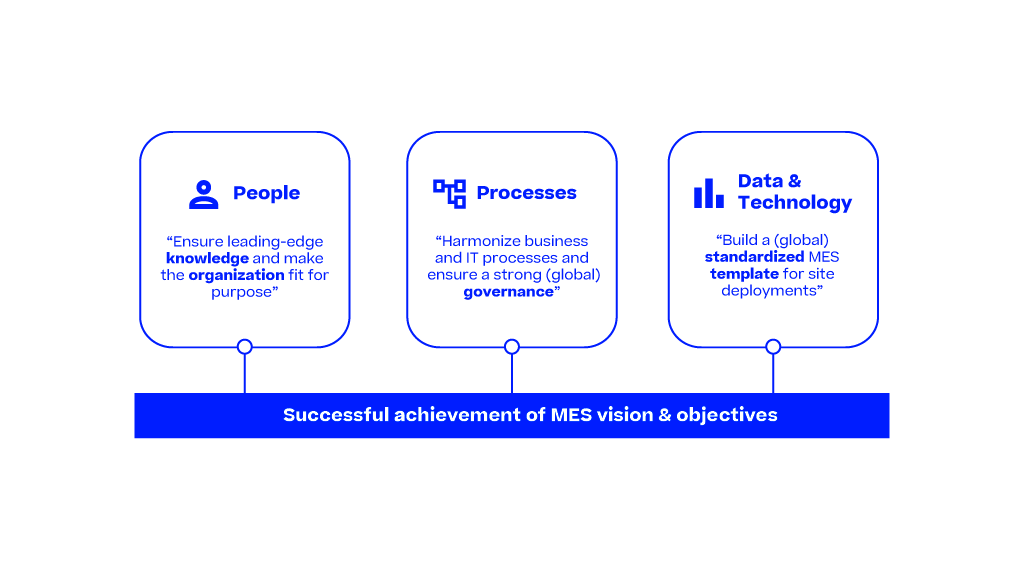
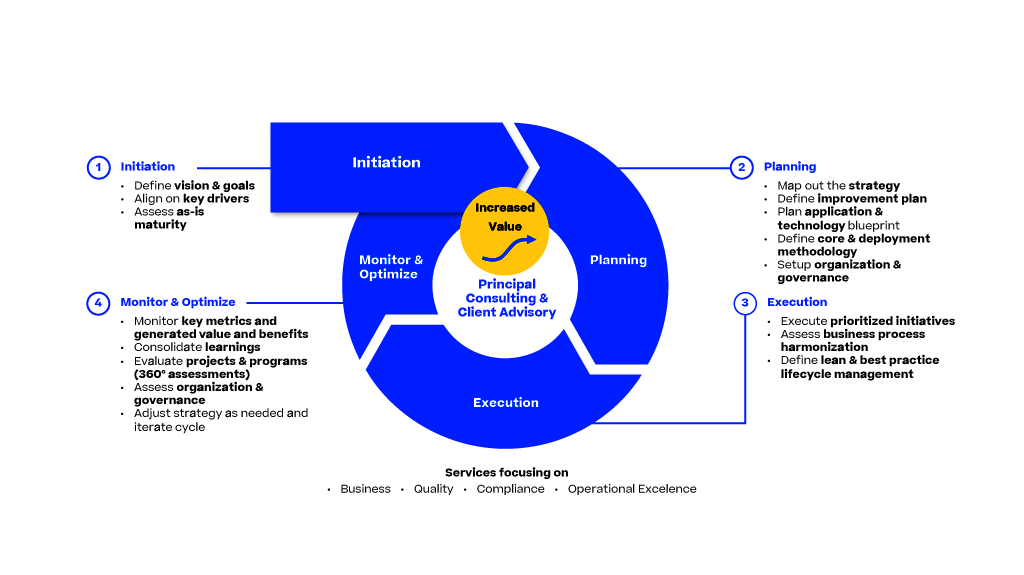
The Pharma 4.0 Strategy gives a high-level introduction to the pharmaceutical industry using advanced digital technologies, such as AI, big data analytics and IoT. This introduction is focused especially on the manufacturing part using PAS-X MES to achieve a digital transformation, enabling smart manufacturing for faster production and optimized supply chains, operational excellence and reducing time to market.