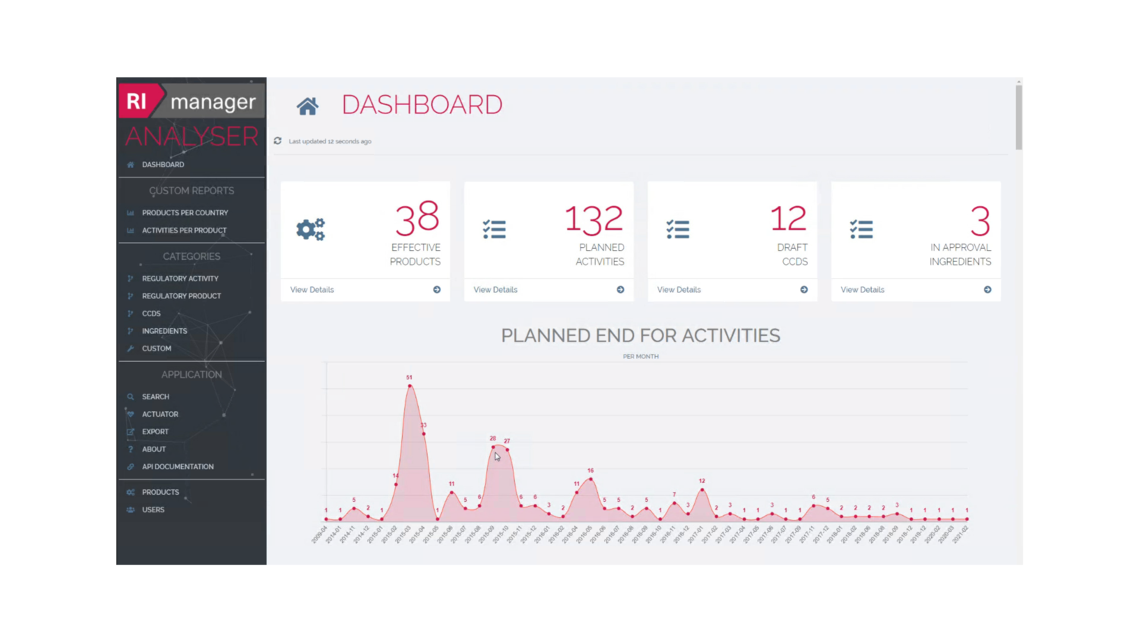
How the RImanager supports you: Managing regulatory projects efficiently
In addition to regulatory data, RImanager includes powerful project management tools that help you structure and steer higher-level regulatory processes.
Integrated task management tools enable you to assign specific activities to individual departments or teams. Direct links between tasks and production sites, pharmaceutical products, formulas, and approval documents allow a fast and targeted access to related information.
For very complex processes, a workflow function can be used to assign specific tasks to different users. The fully audited workflows can also be used to manage review and approval processes. E-mail notifications ensure that users are informed in real time about upcoming tasks and deadlines.
RImanager also contains a version control feature that allows to track the status of every document within the system. For example, you can work on a new draft without changing the published version.
The services at a glance:
- Assignment of tasks and responsibilities to individual team members
- Easy creation of management reports
- Fast and simple creation of product and approval documentation
- Supports EU and US regulatory procedures
- E-mail notifications compliant with FDA CFR 21, Part 11
- Powerful project management tool for planning and tracking regulatory activities, tasks, and resources
- Provision of structured data that supports IDMP-compliant exchange (Identification of Medicinal Products) with the authorities