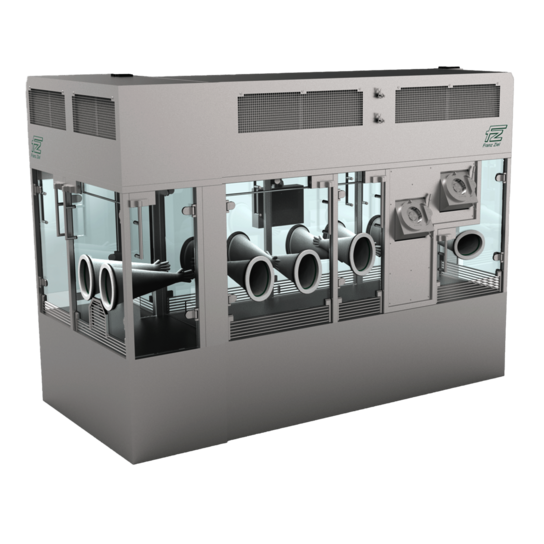
Minimize contamination risk with flexible aseptic processing solutions
Ensure aseptic conditions, operator safety, and regulatory compliance in pharmaceutical and biopharmaceutical production with our multi-layered aseptic processing solutions. We offer you various isolators and Restricted Access Barrier Systems (RABS) as well as cutting-edge laminar air flow systems and material transfer solutions. Within our portfolio you will find everything your production needs.