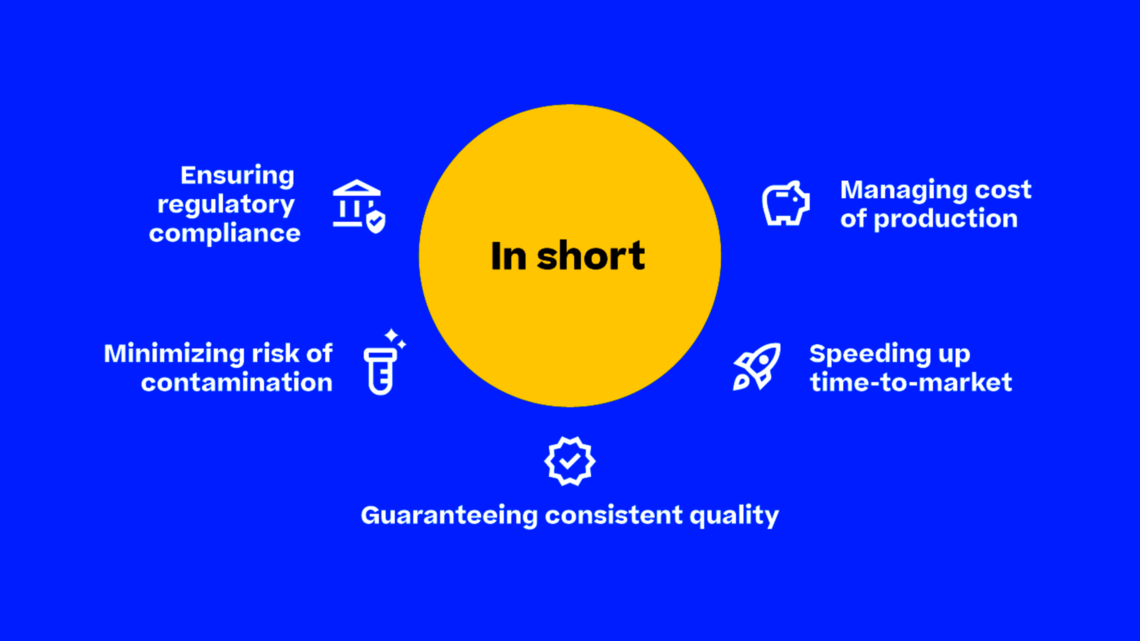
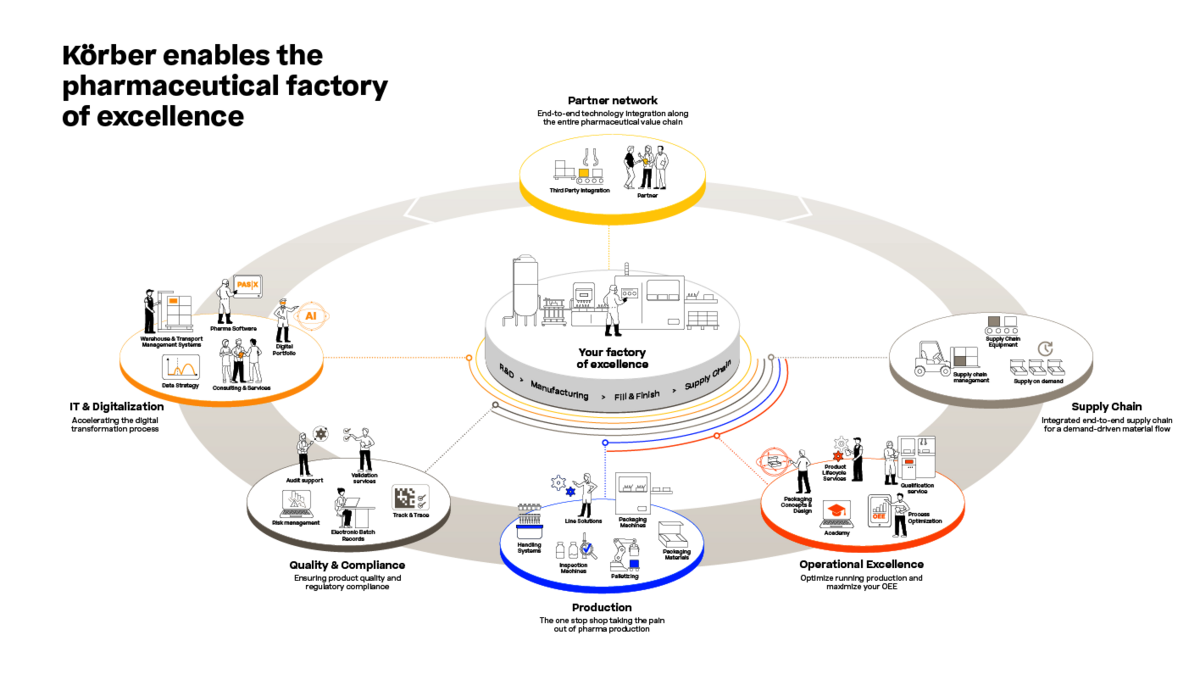
With stringent regulatory requirements across large batch sizes, maintaining compliance can be challenging. Here, Körber's powerful and market-leading PAS-X MES Suite comes into play, digitalizing and automating documentation and reporting tasks, ensuring each of your batches adheres to Good Manufacturing Practice (GMP) standards.