In the dynamic landscape of pharmaceutical manufacturing, the integration of cutting-edge technologies is reshaping industry standards. In this article, we unravel the groundbreaking potential of end-to-end digital twins, also known as Integrated Process Models (IPM), that are redefining the (bio-)pharmaceutical industry's future. From enhanced manufacturing flexibility to accelerated time-to-market, we illustrate how these dynamic tools redefine decision-making in real-time. Additionally, we explore the synergy achieved by integrating digital twins with MES software, unlocking opportunities for real-time batch control and release.
Digital twins were first defined in 2002 by Dr. Michael Grieves at the University of Michigan. Over the past few years, this term has gained widespread attention across numerous industries, including the pharmaceutical industry. But what exactly is a digital twin, and how are they revolutionizing the pharmaceutical landscape?
There are many definitions of digital twins available, but one that particularly resonates with us is the one put forth by IBM¹. According to their definition, a digital twin is described as a “virtual representation of an object or system that spans its lifecycle, is updated from real-time data, and uses simulation, machine learning, and reasoning to enhance decision making". IBM’s perspective emphasizes that digital twins are more than just extensive simulation models. They are dynamic tools, which continuously learn from new data, predict future outcomes, and suggest preemptive actions - vastly amplifying efficiency, reducing risks, and enhancing overall performance.
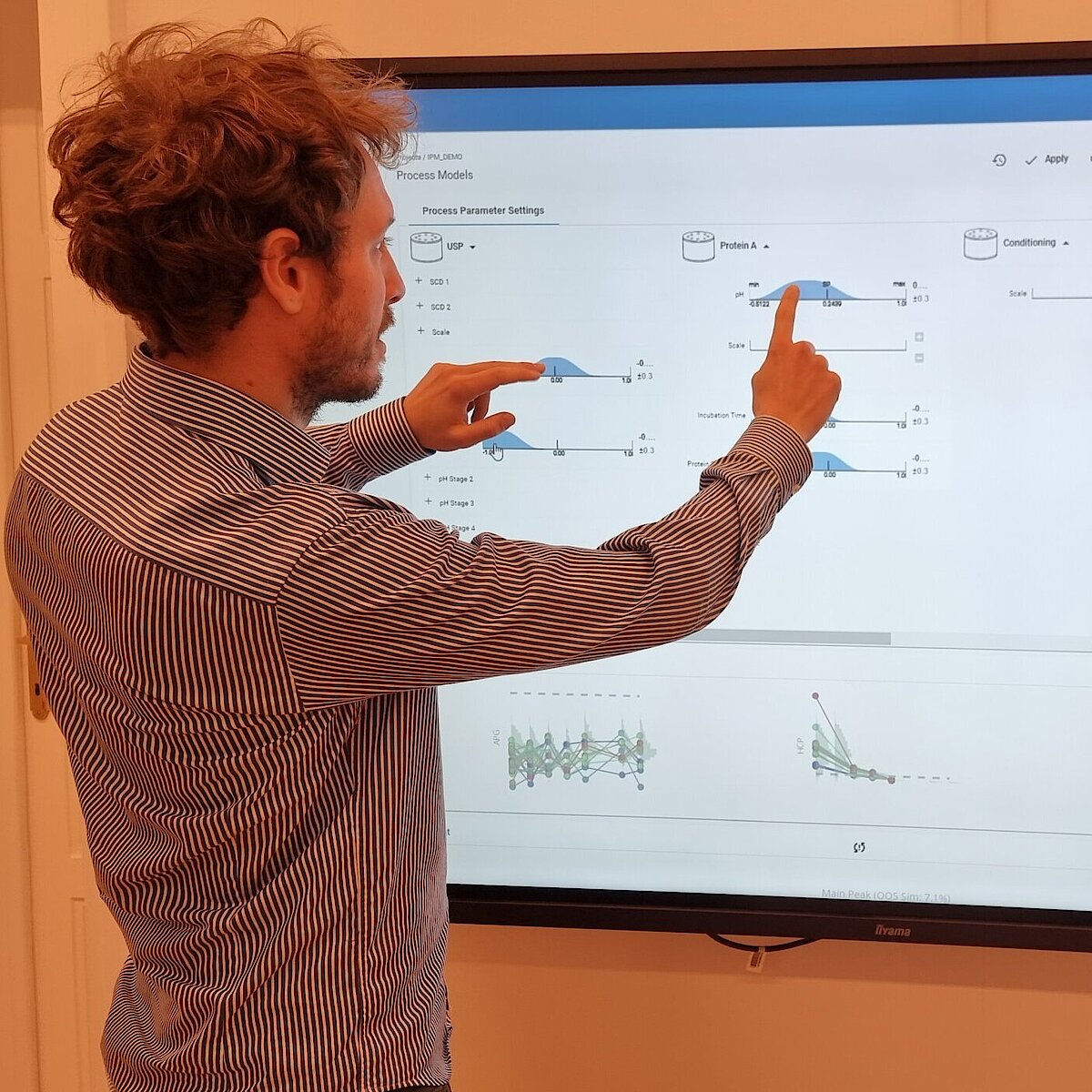
In the realm of process modeling and manufacturing digital transformation, digital process twins can be either specialized for specific unit operations or be holistic, spanning the full spectrum of the production journey. This article will delve into the benefits of the latter approach, known as end-to-end digital twins or Integrated Process Models (IPM), in the biopharmaceutical industry.
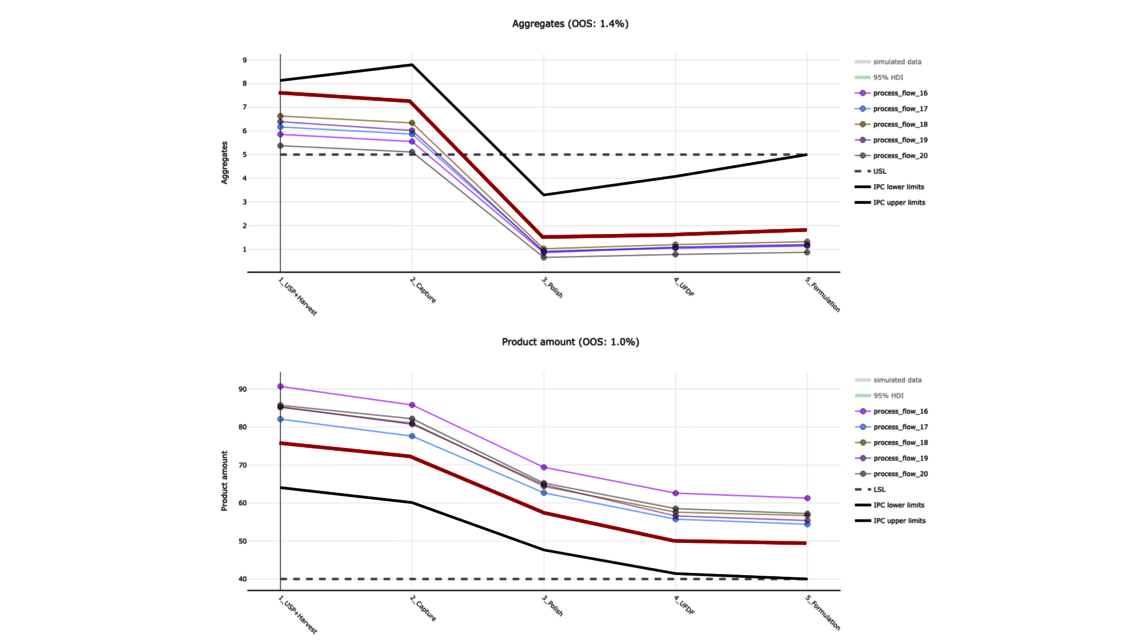
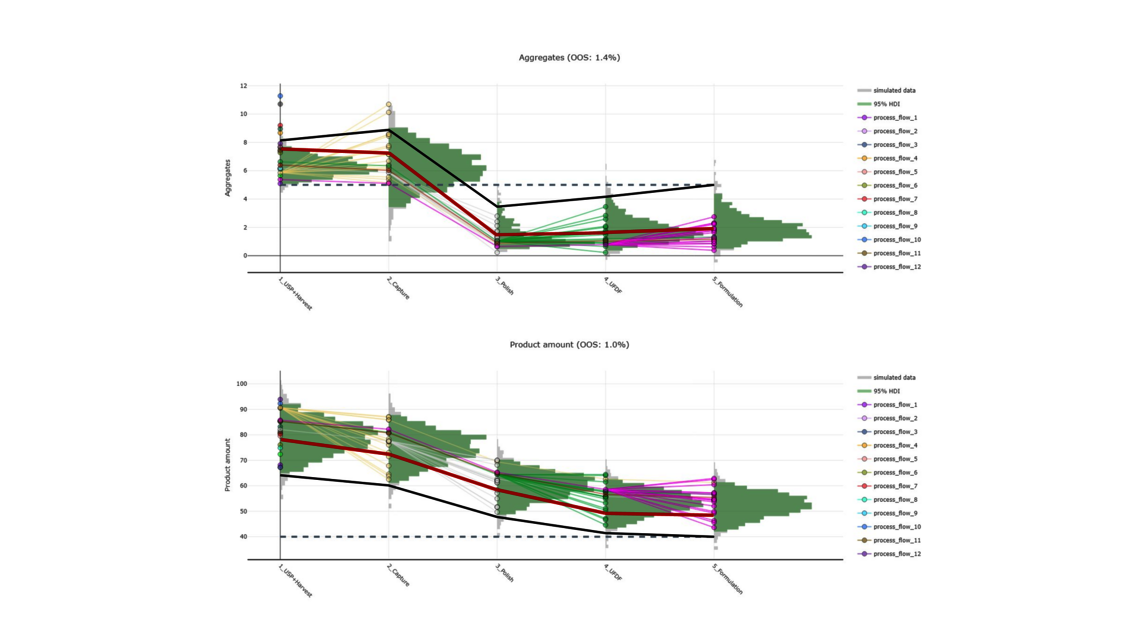
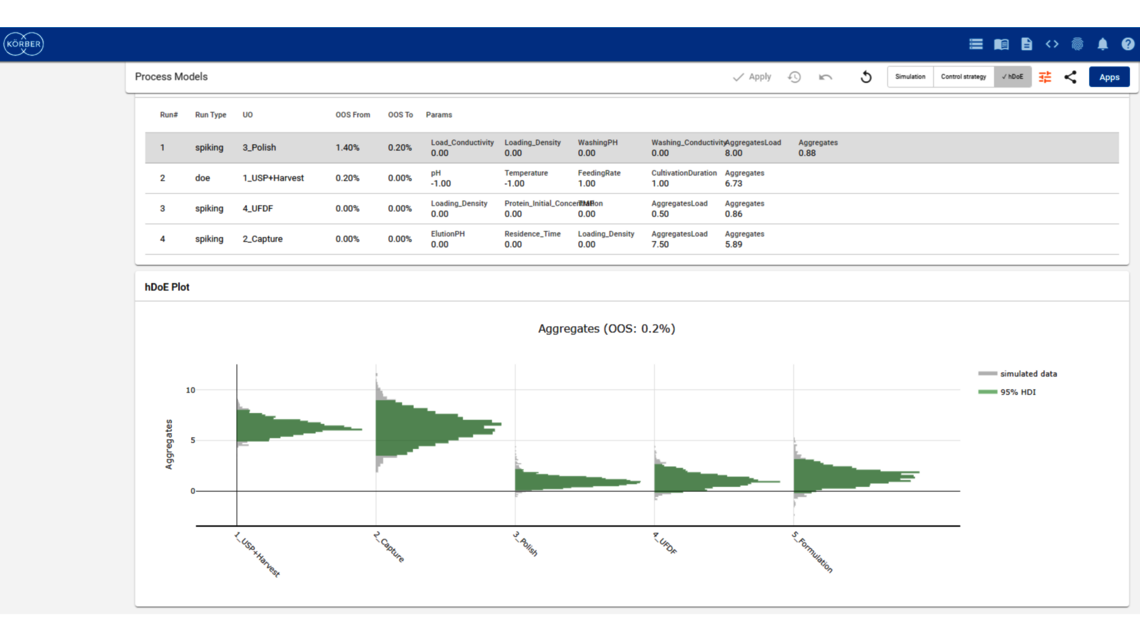
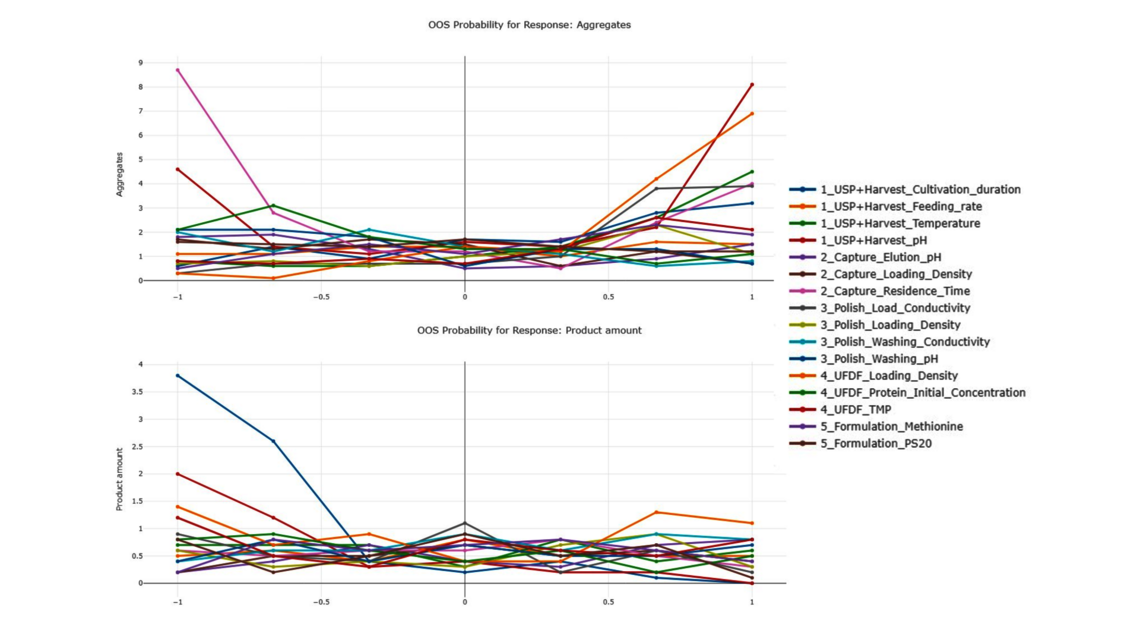
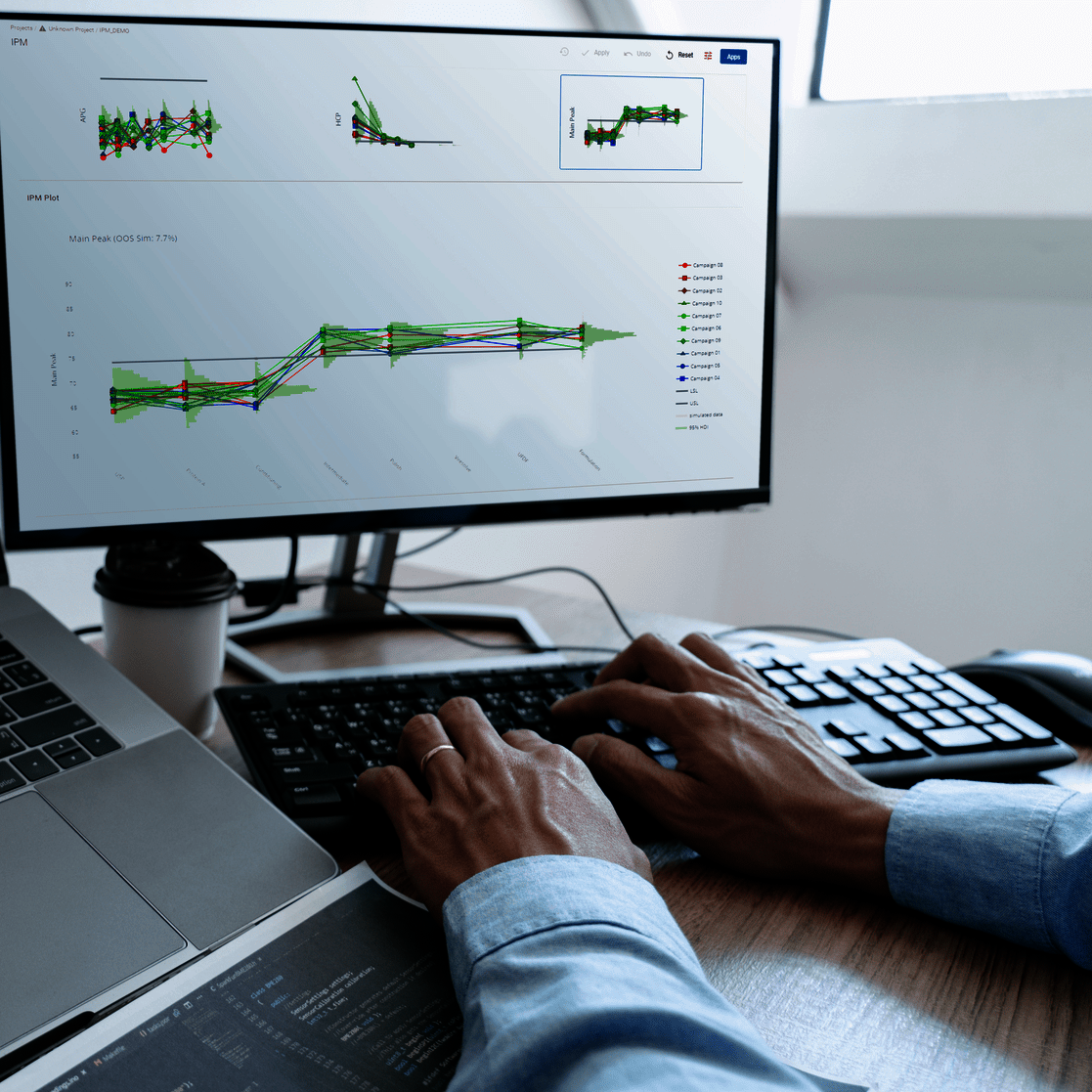
The goal of any biopharmaceutical manufacturing process is to ensure product efficacy and patient safety on a continuous basis while maximizing yield. This is defined by the quality of the drug substance/drug product achieved at the end of the process. However, biopharmaceutical manufacturing processes typically consist of five to ten different unit operations. Therefore, how can the performance of intermediate unit operations be linked to the final product quality? How can we identify the unit operations that have the greatest impact on product quality, enabling us to allocate more resources accordingly? Do we necessarily require models of equal accuracy for all unit operations? These critical questions can only be addressed by adopting a holistic approach that encompasses the manufacturing process from end-to-end, integrating all unit operations under a unified model.
To demonstrate the benefits of end-to-end digital twins in this blog entry, a demo dataset representing a simplified industrial monoclonal antibody (mAb) production process will be used. The dataset comprises five large-scale campaigns across five different unit operations (UOs) and 15 Design of Experiment (DoE) runs per UO, a common dataset size during process validation stage 1 in the biopharmaceutical industry. Two responses will be modeled: Aggregates (a Critical Quality Attribute, CQA) and Product amount (a Key Performance Indicator, KPI).