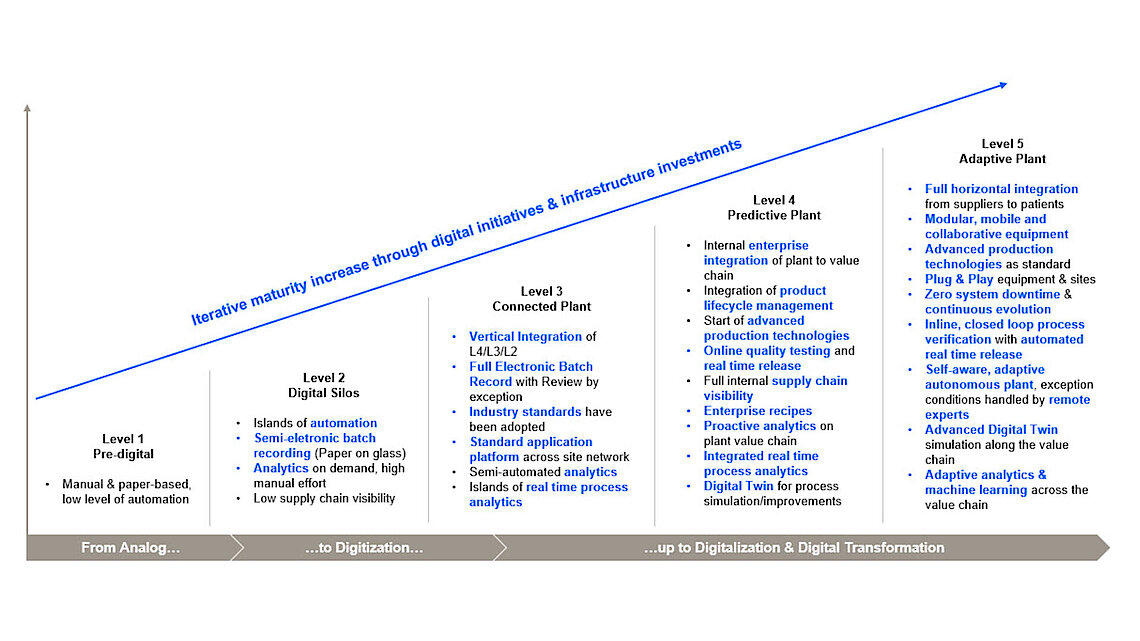
While the pharmaceutical industry is measured by its commitment to improve patients' quality of life, manufacturers simultaneously face an increasingly complex situation.
Among the key requirements are safeguarding and tracing supply chains, maintaining consistently high product quality and data integrity, ensuring smooth process development and technology transfer, accelerating time to market, as well as coping with persistent cost pressures. The industry must evolve to meet these challenges, managing the complexity of drug manufacturing, and serving patients even better.