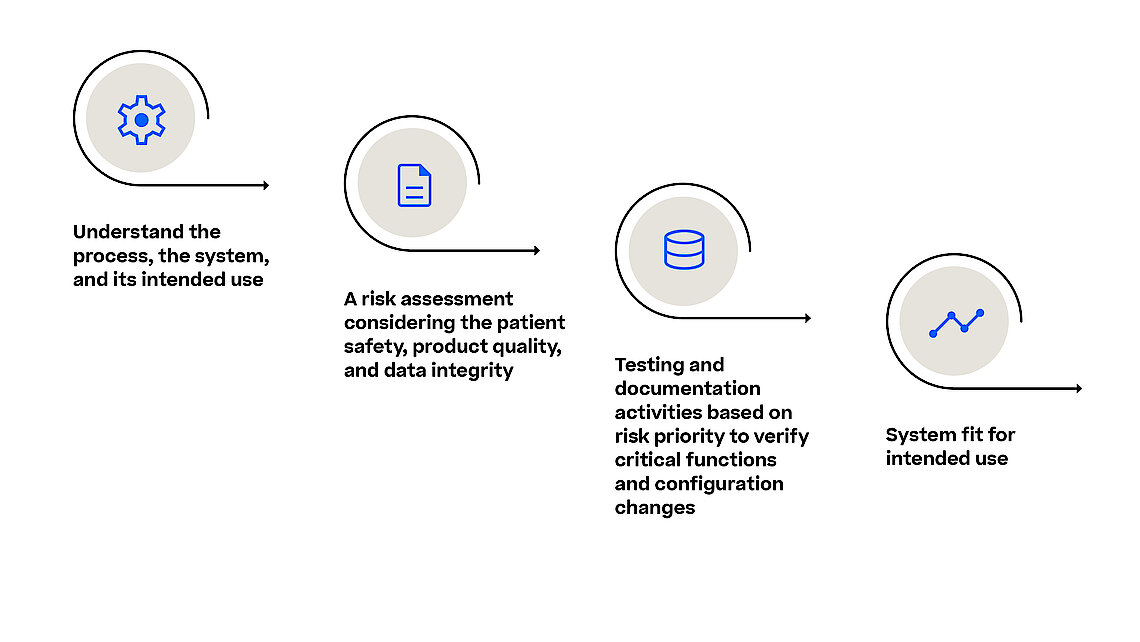
Life sciences companies have been conducting Computerized System Validation (CSV) in accordance with the Food and Drug Administration (FDA) validation guidance. The objective is to ensure that systems employed in the manufacturing of regulated drug products and medical devices fulfill their intended purposes and adhere to data integrity requirements. For regulated companies, ensuring that computer systems meet the criteria for functionality, patient safety, product quality, and data integrity is imperative.
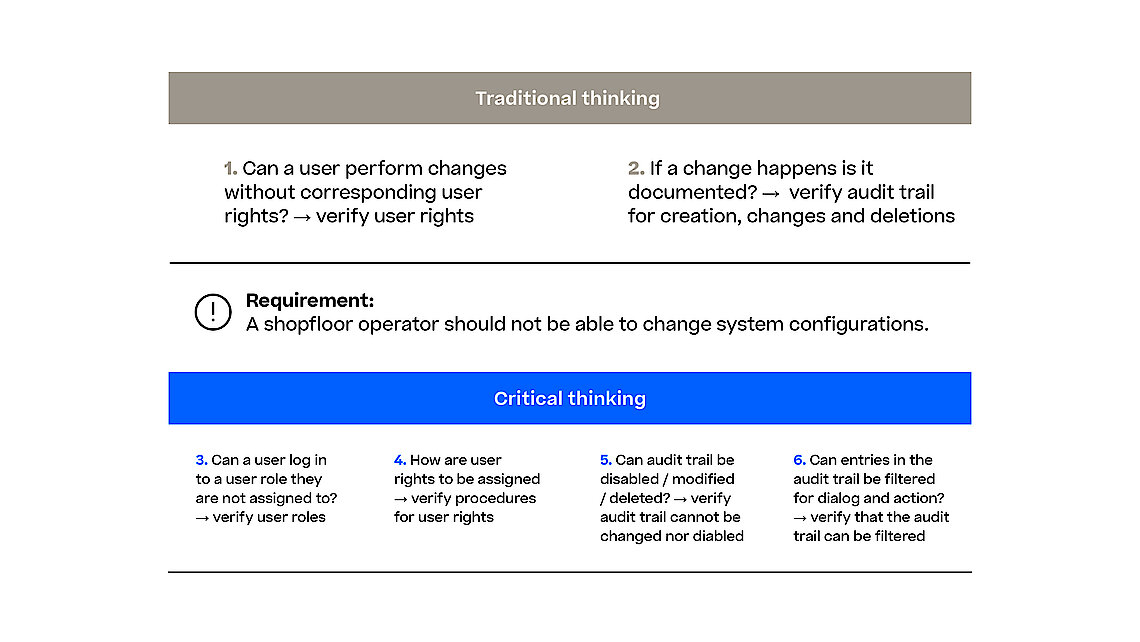
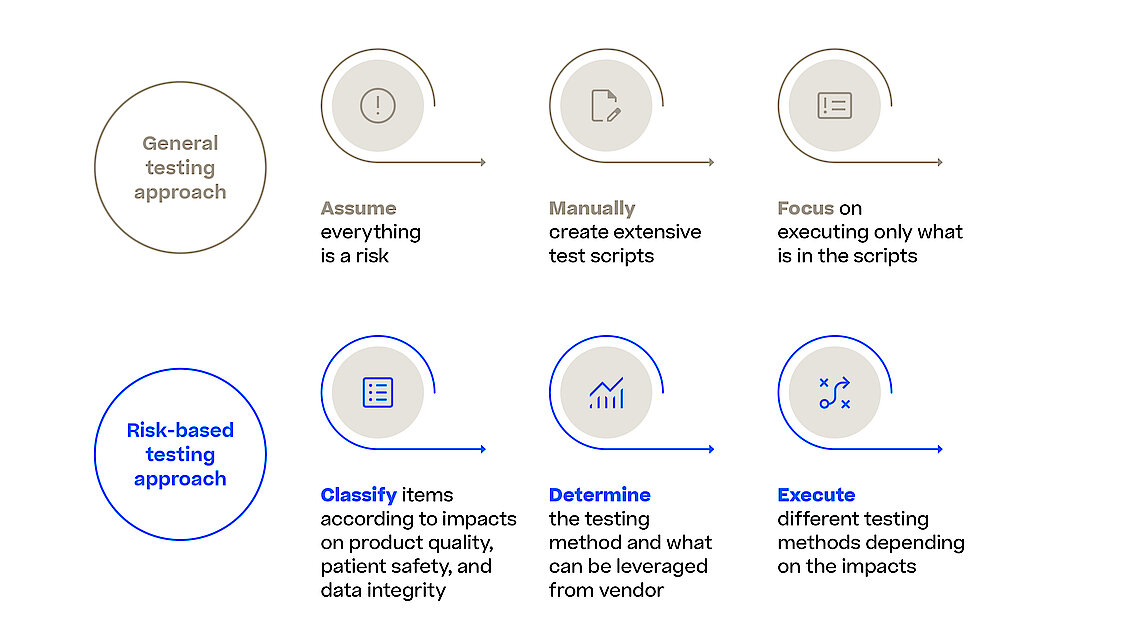
With the Computer Software Assurance (CSA) draft guideline the FDA expands on the outlined principles for software validation from the FDA’s Software Validation guidance and urges the industry to focus on the most relevant and thus risky items in their validation efforts. This is also true when it comes to assuring the reliability, security, and compliance of software systems used in the manufacturing process. Manufacturing execution system (MES) orchestrates and monitors various manufacturing processes (e.g., pharma, biotech, advanced medicine) and any software malfunction or security breach could result in severe quality control issues, regulatory non-compliance, and potentially harm to patients.