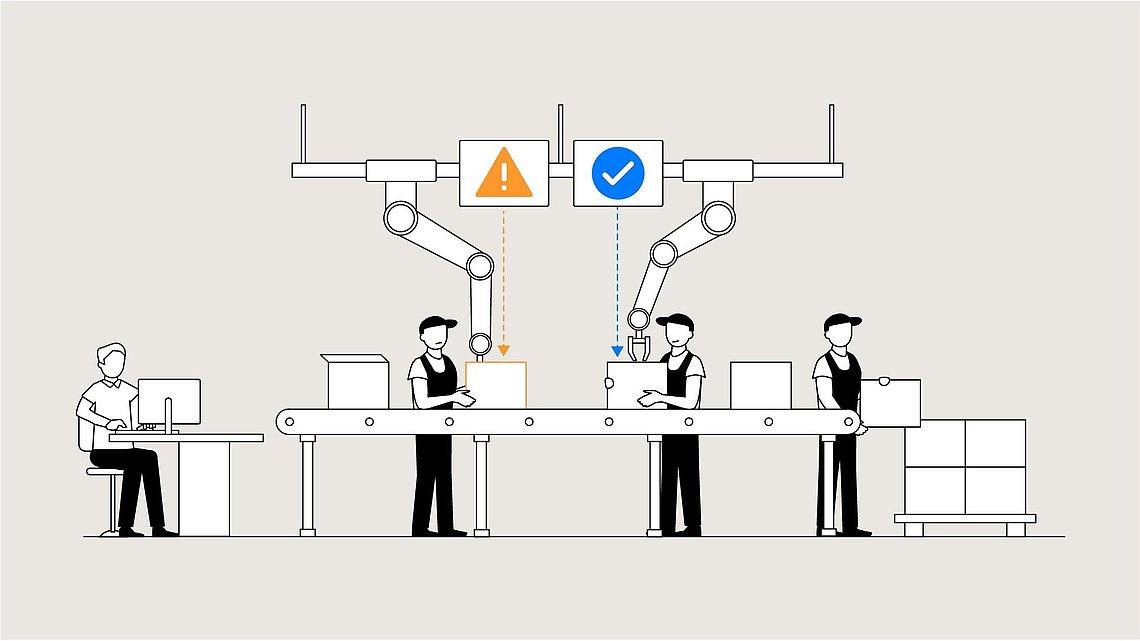
This analogy can be applied to biopharmaceutical processes. We replace the workers here on the conveyor belt by unit operations. The same principle still applies: if we understand which unit operation(s) produces poor product quality we can save runs on all the previous unit operations.
It is as simple as that!
To do this conceptually, we need to employ two types of experiments:
A. Experiments that have been conducted at a specific unit operation to understand the impact of its PPs (Process Parameters) on the product quality delivered by that unit operation
B. Experiments that help to understand how much the input material quality impacts on the product quality measured after the unit operation
In the past we focused only on the first type of experiments (type A). The reasons for this may be due to specific company business practices as different unit operations are handled by different people and departments. This leads to silos of data and knowledge.
Isolated clearance and spiking studies (type B) have also been conducted. However, this data has not been generated and analysed systematically, in addition the relevant information has not been evaluated together with type A experiments of process development at later stages, i.e. process characterization. This may also be due to another aspect of company business practice that leads to this information gap between the stages of the product life cycle.